Fe2O3 Never Sleeps
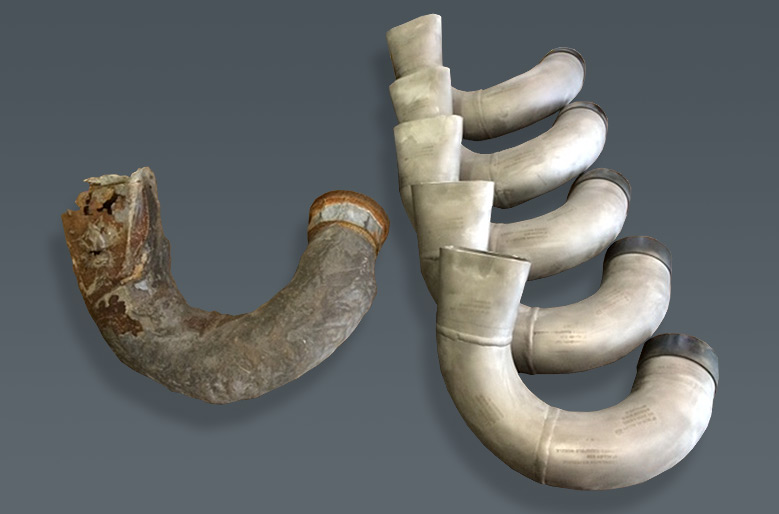
Normally when someone hears about corrosion, they think it is a bad thing. This is true in the case when steel “rusts” or creates an iron oxide on its surface. This exposes the metal and subjects it to further corrosive attack. The oxide is created when the iron in the alloy reacts with oxygen and moisture in the environment. The “rust” expands and is flaky and porous. More oxygen and moisture further react with the iron and expose more of the parent metal, eventually resulting in a failure or total destruction of the metal. This layer is considered as “active.”
In some cases corrosion products like oxides can actually help protect an alloy. When elements like aluminum and chromium are alloyed into a metal, they can create ultra-thin passive layers that actually protect the parent material from further corrosion. These layers are usually oxides that form spontaneously when they come in contact with air. If these barriers are damaged mechanically, it will repair itself immediately. This can be seen in stainless steels and nickel alloys, where a chromium oxide layer can form and provide a barrier against further oxidation.
