
HEAT TREATING NICKEL ALLOYS
Heat treatments are done to alloys and metals to change the properties to fit certain situations. Not all heat treatments are created equal. Because each metal has its own composition, heating and cooling operations performed on the metals to alter their properties happen at different temperatures. Heat treating Fe based alloys is different from treating Ni based alloys. Nickel is an austenite former and these alloys stay austenitic from melting to absolute zero. No phase transformations occur. While some carbides and other precipitates like gamma prime phase can form and harden the alloy, it stays as austenitic.
Almost all heat treatments used on nickel alloys are done to soften the alloy or to increase the strength in the precipitation hardenable alloys. The purposes of heat treatments are:
- Remove stresses introduced into the alloys during cold work
- Restore ductility
- Reduce tensile properties
- Strengthen the material
Depending on the design and end use combined with the exact chemical composition of the alloy, several types of heat treatment of nickel alloys are recommended:
- Stress equalizing is performed at lower temperatures to significantly balance stresses in cold worked material with decreasing mechanical strength.
- Stress relieving is used to remove or reduce stresses in work hardened alloys. The stresses are relieved without causing recrystallization in the grains by heating to 800-1600°F.
- Annealing is when an alloy is heated (usually to 1300-2200°F), the temperature is maintained for a while, then cooled. This treatment recrystallizes the grain structure. Annealing totally eliminates any stresses in the material, causes reduced tensile strength and improves ductility.
- Solution annealing is a high temperature anneal (2100-2400°F based on composition) specific to certain alloys. It puts any carbides that have formed completely back into the solution and creates coarse grain size to enhance stress rupture properties.
- Age hardening uses heat (800-1600°F) to change the solubility of an alloy that contains elements that promote this. Aging causes certain phases to precipitate at certain temperatures to increase strength. For this precipitation to happen, alloys must be kept at these high temperatures for extended amounts of time. This extended time is what is referred to as “age”.
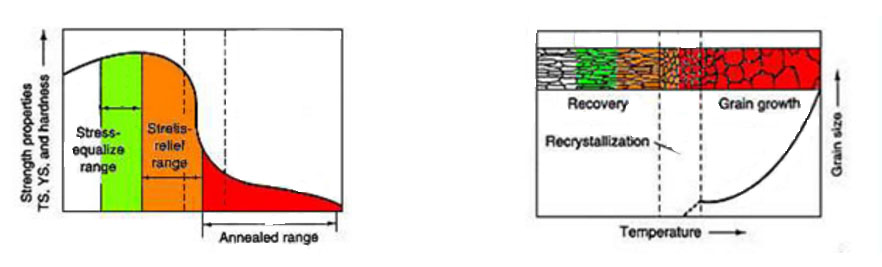
◊ Schematic representation of the effect of annealing temperature on the properties and grain structure/size of nickel alloys. TS: tensile strength; YS: yield strength.
